Introduction
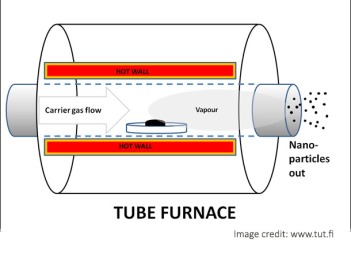
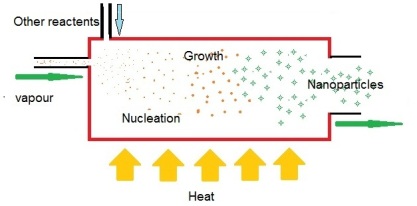
Gas phase nanoparticle preparation methods have attracted huge interest over the years due to number of benefits that they can deliver over other methods. These techniques are typically characterized by the ability to accurately control the process parameters to be able to tune shape, size and chemical composition of the nanostructures.
Although, means and methods can differ, almost all gas phase nanomaterial production methods follows following sequence
-
Suspending the precursor materials in a gas phase
-
Transforming the precursor material to small clusters
-
Enforcing the growth of these clusters to a nanoparticles
-
Method to collect prepared nanoparticles.
Thank you for your clear explanations on the gas phase synthesis of nanoparticles. It was very useful in informing my undergraduate report on nanoparticles. Best of luck to you!
LikeLike
you have mention in inert gas condensation process that this process is carried out for materials that have low vapor pressure instead of high…..
LikeLike